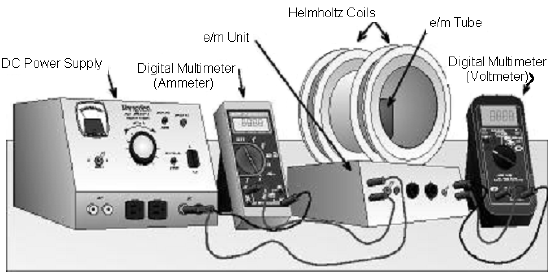
Following experiment was performed by J.J. Thomson in order to measure ratio of charge e and mass m of electron. Electrons emitted from a hot filament - Sarthaks eConnect | Largest Online
In a hydrogen atom an electron of mass m and charge e revolves in an orbit of radius r making n revolutions per second if the mass of hydrogen nucleus is M

The 'm' value an electron in an atom is equal to the number of m values l =1. The electron may be present in:
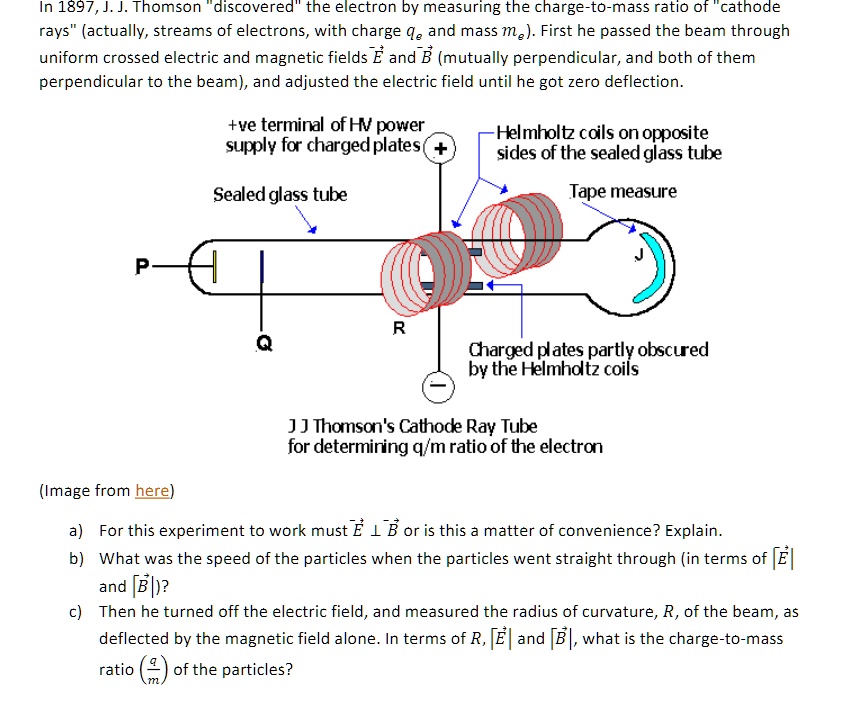
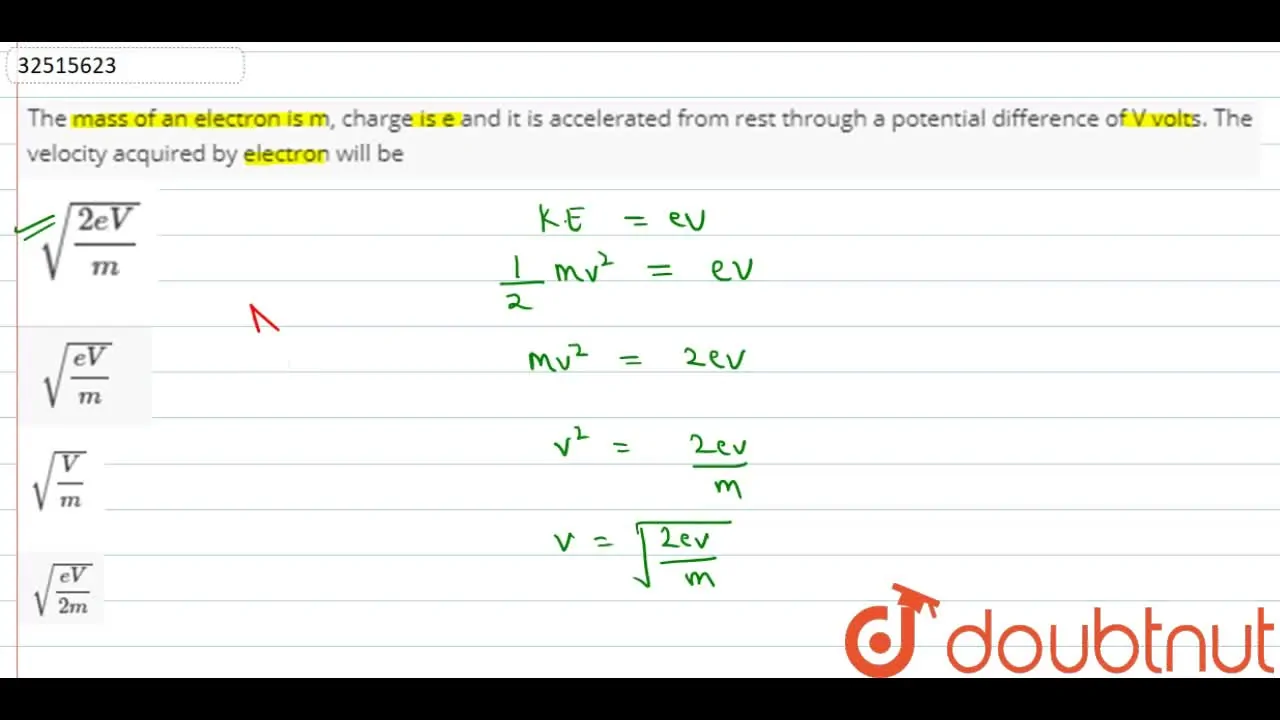
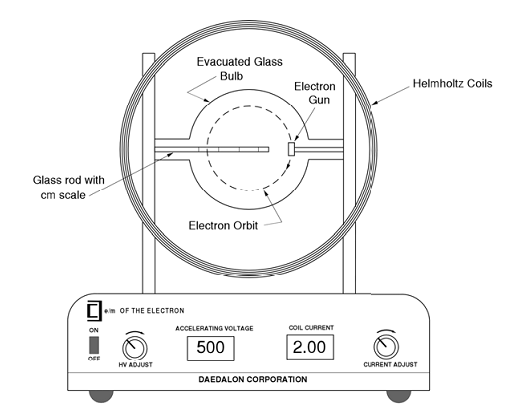
![PDF] Electron Charge to Mass Ratio e / m | Semantic Scholar PDF] Electron Charge to Mass Ratio e / m | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/0df2f9e9179fc25cfae0c4c14e756a609e820545/3-Table1-1.png)





![Solved] How many electrons are present in M - Shell of an element wi Solved] How many electrons are present in M - Shell of an element wi](https://storage.googleapis.com/tb-img/production/22/03/F1_Shraddha_Neha%20G_17.12.2021_D1_Corrected%201.png)